Synthetic chemistry is inseparable from solvents, such as reaction media, HPLC column purification as eluent, extraction, etc. TLC spot plates require us to configure the unfolding agent, which then requires us to understand the nature and polarity of the various solvents.
Polarity
Polarity is the degree to which the entire molecular charge is separated. The greater the separation, the greater the polarity. So usually, molecules containing electron-absorbing groups such as N, O, and halogens will be more polar, but it should be noted that chloroform is more polar than dichloromethane because it has extra electron-absorbing chlorine. Still, carbon tetrachloride is less polar than chloroform because it is a symmetrical structure, and the polarity of a compound is determined by the functional groups in the molecule and the molecular structure. The effect of the group on the polarity of the substance should be related to the structure of the substance, and it cannot be said that the group on which the polarity increases or decreases should be analyzed as a whole.
Alkanes are the least polar because the C and H in them are about the same electronegativity and the charge separation is insignificant.
Olefins have double bonds and are more polar than alkanes. Some books say that double bonds are electron-absorbing, so charges are separated. I think this is only explained in terms of the end effect, and theoretically, it could be due to the super conjugation effect formed by the double bond with the surrounding C-H bond causing the electrons to be biased towards the double bond.
A discussion of the relationship between functional group conversion and polarity
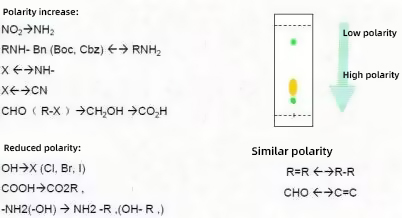
The details are summarised below.
The following diagram shows the order of polarity of mixed organic solvents (from small to large, the proportion of the mixture is indicated in parentheses) cyclohexane-ethyl acetate (8+2) → chloroform-acetone (95+3) → benzene-acetone (9+1) → benzene-ethyl acetate (8+2) → chloroform-ethyl ether (9+1) → benzene-methanol (95+5) → benzene-ethyl ether (6+4) → cyclohexane-ethyl acetate (1+1) → chloroform-ethyl ether (8+2) → azoxymethane-methanol (99+1) → benzene-methanol (9+1) → chloroform-acetone (83+15) → benzene-ethyl ether (4+6) → benzene-ethyl acetate (1+1) → chloroform-methanol (95+3) → chloroform-acetone (7+3) → benzene-ethyl acetate (3+7) → benzene-ethyl ether (1+9) → ether-methanol (99+1) → ethyl acetate-methanol (99 +1) → benzene – acetone (1+1) → chloroform-methanol (9+1)
Strong polar solvents: methanol > ethanol > isopropanol Medium polar solvents: ethyl cyanide > ethyl acetate > chloroform > methylene chloride > ethyl ether > toluene Non-polar solvents: cyclohexane, petroleum ether, hexane, pentane
Organic solvent polarity table
|
Compound name |
Polarity |
Viscosity |
Boiling point |
Absorption wavelength |
|
i-pentane |
0 |
- |
30 |
- |
|
n-pentane |
0 |
0.33 |
36 |
210 |
|
Petroleum ether |
0.01 |
0.3 |
30~60 |
210 |
|
Hexane |
0.06 |
0.33 |
69 |
210 |
|
Cyclohexane |
0.1 |
1 |
81 |
210 |
|
Isooctane |
0.1 |
0.53 |
99 |
210 |
|
Trifluoroacetic acid |
0.1 |
- |
72 |
- |
|
Trimethylpentane |
0.1 |
0.47 |
99 |
215 |
|
Cyclopentane |
0.2 |
0.47 |
49 |
210 |
|
N-heptane |
0.2 |
0.41 |
98 |
200 |
|
Butyl chloride |
1 |
0.46 |
78 |
220 |
|
Trichloroethylene |
1 |
0.57 |
87 |
273 |
|
Carbon terachloride |
1.6 |
0.97 |
77 |
265 |
|
Trichlorotrifluoroethane |
1.9 |
0.71 |
48 |
231 |
|
i-propyl ether |
2.4 |
0.37 |
68 |
220 |
|
Toluene |
2.4 |
0.59 |
111 |
285 |
|
p-xylene |
2.5 |
0.65 |
138 |
290 |
|
Chlorobenzene |
2.7 |
0.8 |
132 |
- |
|
o-dichlorobenzene |
2.7 |
1.33 |
180 |
295 |
|
Ethyl ether |
2.9 |
0.23 |
35 |
220 |
|
Benzene |
3 |
0.65 |
80 |
280 |
|
Isobutyl alcohol |
3 |
4.7 |
108 |
220 |
|
Methylene chloride |
3.4 |
0.44 |
40 |
245 |
|
Ethylene dichloride |
3.5 |
0.78 |
84 |
228 |
|
n-butanol |
3.7 |
2.95 |
117 |
210 |
|
n-butyl acetate |
4 |
- |
126 |
254 |
|
n-propanol |
4 |
2.27 |
98 |
210 |
|
Methyl isobutyl ketone |
4.2 |
- |
119 |
330 |
|
Tetrahydrofuran |
4.2 |
0.55 |
66 |
220 |
|
Ethyl acetate |
4.30 |
0.45 |
77 |
260 |
|
i-propanol |
4.3 |
2.37 |
82 |
210 |
|
Chloroform |
4.4 |
0.57 |
61 |
245 |
|
Methyl ethyl ketone |
4.5 |
0.43 |
80 |
330 |
|
Dioxane |
4.8 |
1.54 |
102 |
220 |
|
Pyridine |
5.3 |
0.97 |
115 |
305 |
|
Acetone |
5.4 |
0.32 |
57 |
330 |
|
Nitromethane |
6 |
0.67 |
101 |
330 |
|
Acetic acid |
6.2 |
1.28 |
118 |
230 |
|
Acetonitrile |
6.2 |
0.37 |
82 |
210 |
|
Aniline |
6.3 |
4.4 |
184 |
- |
|
Dimethyl formamide |
6.4 |
0.92 |
153 |
270 |
|
Methanol |
6.6 |
0.6 |
65 |
210 |
|
Ethylene glycol |
6.9 |
19.9 |
197 |
210 |
|
Dimethyl sulfoxide |
7.2 |
2.24 |
189 |
268 |
|
Water |
10.2 |
1 |
100 |
268 |

Commonly used solvent mixes
1) Ethyl acetate/hexane: commonly used in 0 to 30% concentrations. However, removing the solvent completely on a rotary evaporator is sometimes difficult.
2) Ether/pentane system: concentrations of 0-40% are more commonly used. Very easy to remove on a rotary evaporator.
Ethanol/hexane or pentane: 5 to 30% is more suitable for strongly polar compounds.
Dichloromethane/hexane or pentane: 5~30%, may be considered when other solvent mixtures fail.
3) Pour 1 to 2 mL of the selected solvent system into the unfolding cell and place a large piece of filter paper in the cell.
4) Spot the compound at the marked baseline. The spotter we used was purchased. In addition, the spotter can also be pulled off a heated Pasteur pipette (you can refer to UROP). When following the reaction as it proceeds, spot the starting reactant, the reaction mixture, and the mixture of the two.
5) Unfold: Allow the solvent to unfold upwards for approximately 90% of the length of the sheet.
6) Remove the sheet from the unfolding cell and immediately mark with a pencil where the solvent reaches the front. Calculate the value of Rf from this.
7) Allow the solvent to evaporate from the sheet.
8) Observe the sheet using a non-destructive technique.
The best non-destructive method is to use an ultraviolet lamp for observation. Place the sheet under the UV lamp and mark all points with UV activity with a pencil. Although this method is not used in 5.301, we will use another commonly used non-destructive method – staining with iodine.
9) destructively observe the sheet.
This method can only be used when the compound has no UV activity. Several very useful stains are provided in 5.301. To use the stain:
- Please pick up a dry, thin plate with tweezers and place it into the stain, submerging it from the baseline to the solvent front.
- Dry the back of the plate with a paper towel.
- Place the plate on a heating plate and observe the changes in the spots.
- Remove the plate from the heating plate before the spots become visible and the background color fails to cover the spots.
10) Modify the choice of the solvent system based on the initial thin-layer chromatography results. If you want to make the Rf larger, make the solvent system more polar; if you want to make the Rf smaller, make the solvent system less polar. If the spot sample on the plate becomes a streak rather than a circle, your sample concentration may be too high. Dilute the sample and perform another thin-plate chromatography; if this still does not work, consider changing the solvent system.
Post time: Oct-13-2022






