The development of HPLC methods is usually time-consuming and laborious. A common approach is to use the customary combination of solvents, pH conditions, buffer salts, and commonly used hplc columns as starting conditions and then make a series of adjustments to the mobile phase ratios until a more satisfactory peak shape and separation is obtained. However, this is usually extremely time-consuming and only results in optimal separation conditions.
A more systematic methodological strategy is to use a good experimental design and follow the following process to develop an efficient, high-quality reversed-phase chromatography method. This method development process includes.
Method screening: The mobile phase pH, organic solvent, and column are first screened as the most influential, and therefore most important, condition factors on selectivity.
Method optimization: For all the above results, it was evaluated which combination of these three factors gave the best separation. Optimization or fine-tuning is performed based on this combination of conditions to obtain the final desired results.
Method validation: The final method is validated to check whether it satisfies the desired separation effect and analytical purpose.
PART 01
Initiation
In order to design an effective test method mapping experiment, it is important to gather as much information as possible about the chemical properties of the sample and the analyte.
>>>> Information needed for method development
– Sample dissolution properties
The number of analytes? How many analyte peaks need to be separated?
– Chemical structure
Functional groups? What is the difference between the analytes? Are there any ionization groups? How do the ionization constant and pH affect the chromatographic analysis?
– Detection
What kind of Detection is needed or possible? Does the analyte have UV absorption, λmax?
– Concentration range and quantification requirements
What level of Detection is required? What limit of quantification is required?
– Sample matrix effects
Are there matrix effects that prevent separation or Detection?
>>>> Purpose of method use
Before developing a test method, it is also important to consider it.
Are the objectives and purpose of using the test method understood and clarified?
Will a clear understanding of the purpose of use help determine the performance parameters that must be met and the key parameters needed to validate the method?
For example, a method may be required to separate an object subject from all components of the sample, or a method may be required to separate the object subject from other components, enabling all components to be separated from each other.
Other considerations may include: How much throughput is needed? How much resolution is needed? Acceptable trailing factors, etc.
An example of method development for paroxetine-related substances is used to demonstrate the system strategy.
PART
02 Method Selection
01 Effect of pH
When the analyte contains ionizable functional groups (ionizable functional groups are those that can be in different degrees of ionization at different pH conditions, e.g., primary/secondary/tertiary amine groups, carboxylic acid groups, phenolic acid groups), pH conditions will strongly influence the reversed-phase retention of the analyte, and changes in pH conditions will also cause the strongest selectivity-altering effects.
Acidic and basic compounds have the greatest reversed-phase retention when in their un-ionized state, and retention of neutral compounds is not affected by pH.
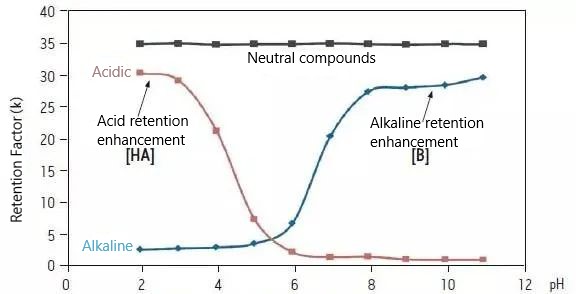
How does pH affect reversed-phase retention properties?
For ionizable compounds, to obtain the strongest reversed-phase retention, the analyte should be as uncharged or ionized as possible when the analyte is relatively less polarized and will be more strongly retained on the reversed-phase column.
Therefore, low pH conditions should be used for acidic compounds, while high pH conditions are more suitable for basic compounds. Neutral compounds do not have any ionizable functional groups and, therefore, are not affected by pH conditions.
These dotted line plots, like titration curves, are highly correlated with the pKa values of the analyte molecules.
When developing a method, avoid screening pH conditions near the pKa value of the compound molecule; otherwise, small changes in pH can lead to dramatic changes in retention and selectivity. The “plateau region” of the retention curve is the more durable region of the analytical method.
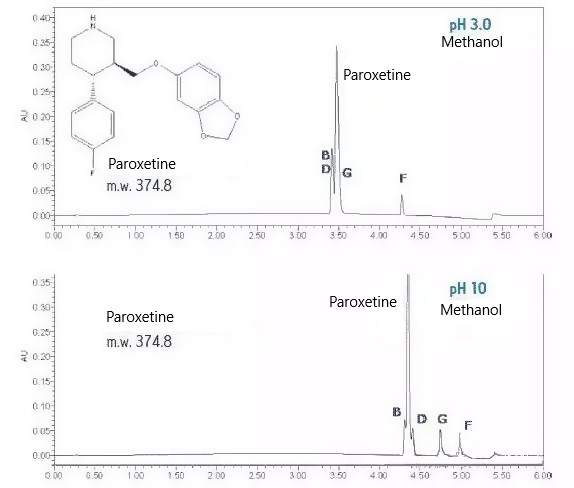
For paroxetine and its related substances B, D, G and F, stronger retention and better separation selectivity can be obtained using pH 10 conditions than pH 3 due to the different ionization states of their amine structures at different pH conditions. Due to the higher resolution and better selectivity, pH 10 is the appropriate pH for the analytes.
The next step was to evaluate the chromatograms of three different columns at the same pH 10 value to select the best resolution. All three columns showed the ability to detect all components, and due to the versatility of C18, the BEH C18 column was chosen to compare the selectivity of the different organic modifiers.
02 Influence of Chromatographic Columns
When selecting chromatographic columns for analytical method development, it should be fully recognized that chromatographic packing matrices (silica particles, hybrid particles, polymer particles) provide different retention and selectivity effects depending on their chemical properties.
Different bonding phases, different selectivity
Hydrophobic – long alkyl chains provide stronger retention
Silyl hydroxyl activity – affects peak symmetry and secondary interactions
Anti-hydrolytic stability – bonding phase (e.g. C18) is bonded to the matrix particles by more bonds
The density of functional groups – affects sample loading
03 Effect of organic solvents
Methanol and acetonitrile are the typical organic solvents used for reversed-phase separations. In addition to elution strength, viscosity (related to column back pressure), and UV absorbance, methanol and acetonitrile differ in their chemoselectivity, with methanol allowing hydrogen bonding and thus providing significantly different selectivity.
Methanol: less eluting, the protonated solvent that provides hydrogen bonding
Acetonitrile: Non-protonated solvent, higher elution capacity, lower viscosity (lower column back pressure)
Relative elution strength curves for organic solvents:
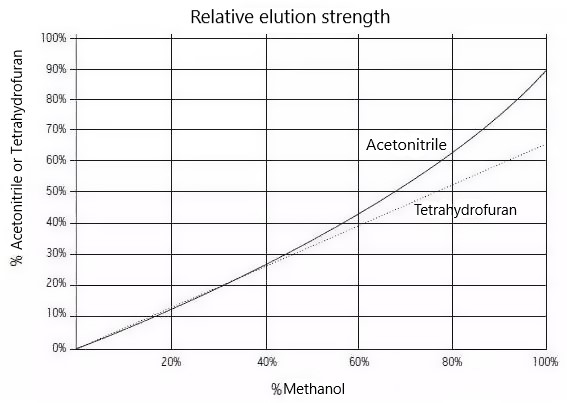
The experimental profiles showed that methanol had a weaker elution capacity than acetonitrile; therefore, stronger retention was obtained for all analytes. However, for this application, acetonitrile provided better separation selectivity. Therefore, the combination of pH 10, column BEH C18 and acetonitrile conditions can be used as the next step in optimizing fine-tuning.
PART
03 Method optimization
Several parameters can be used to adjust or optimize chromatographic separation. The gradient slope is a physical parameter used to control retention and selectivity. However, the gradient slope is often a balance between separation and sensitivity. Another parameter that can be optimized is temperature. Every chemical process is influenced by temperature, which can provide significantly different selectivity.
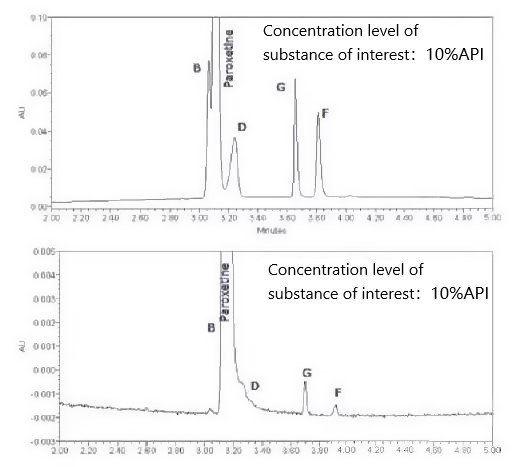
The separation of the relevant substances B and D from the main paroxetine peak was significantly reduced when the concentration of the relevant substances was decreased. This is due to the different concentration levels of the components and paroxetine peaks. Further optimization is required at this point.
>>>> Method optimization and gradient slope
-A slower gradient slope will improve separation
-Lower gradient slopes will reduce sensitivity
-Steeper gradient slopes may compress peaks, often resulting in reduced separation
-Increasing the gradient slope will increase the sensitivity
-Changing the gradient slope balances peak height with separation
-Change retention and selectivity
>>>> Method optimization and temperature
-Reducing mobile phase viscosity
-Reduce back pressure
-If the flow rate remains constant, improve analyte diffusion
-Higher optimized linear velocity
-Change retention and selectivity
Temperature affects every chemical process in between. For example, increasing the temperature will decrease the viscosity of the mobile phase, and if the flow rate is constant will result in lower back pressure of the system. The high temperature will change the partition rate between the stationary and mobile phases. However, the improved analyte dispersion will speed up the optimized linear velocity.
Small temperature changes can result in notable changes in selectivity for compounds sensitive to temperature changes.
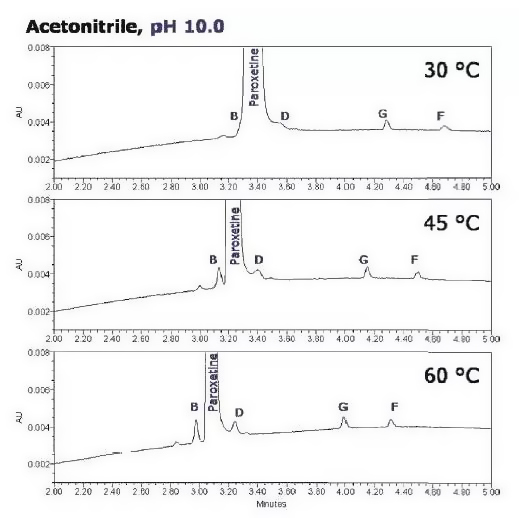
In this example, the temperature was increased in steps of 15 °C from 30 °C to 40 °C and 60 °C. As the column temperature increased, the separation performance of the components improved. In addition, the dispersion of the analytes improved, and the spectral peaks were sharper, with the best separation at the column temperature of 60 °C.
PART
04 Analytical Method Validation
Analytical method validation is often an iterative manual process consisting of multiple steps, requiring extensive chromatographic testing to evaluate all eight properties fully. It can be challenging to review, calculate and manage all the data obtained from these processes. Empower Method Validation Manager uses an automated validation process that allows for smoother data review and greatly improves the efficiency and reliability of the validation process.
Method Validation Tips
Method validation is used to confirm that the characteristics of the analytical method meet the application requirements. Typical chromatographic method characteristics to be evaluated during validation are specificity, linearity, range, detection limit, quantification limit, accuracy, precision, and stability.
PART
05 Conclusion
The chromatographic test method development efficiency can be significantly improved by using a methodology that includes mapping experiments and validation.
Designing experimental studies that include multiple parameters affecting selectivity, such as stationary phase, pH, and organic modifiers, automated runs greatly accelerate the test method development process. After a rational review of the data, completing the final test method is often a process of fine-tuning and optimizing variables such as gradient slope and temperature.
To ensure that the developed method meets the application requirements, the method needs to be validated using the performance parameters determined earlier. Validation Results – The validation of the paroxetine method using the Empower Method Validation Manager are as follows:
|
Parameters |
Qualification criteria |
Reported value |
Accept/reject |
|
Linear |
R2≥0.995 Residuals≤0.2%RSD |
0.9999 1.74%RD |
Accept Accept |
|
Accuracy |
80-120% |
97-102% |
|
|
Intermediate Precision |
Variance components≤10%RSD |
Analysis 1.33%RSD Instrument 7.22%RSD Chromatographic column 0.00%RSD |
Accept Accept Accept |
|
Impurity minimum detection limit |
0.1% of active impurities at 0.2mg/ml |
0.05% active impurity at 0.2mg/ml (S/N 2.2-6.23) |
Accept |
|
Method stability peak area |
Variance components ≤ 2% RSD peak area (buffer strength, additive concentration,, column temperature) |
0.06-1.64%RSD |
Accept |
|
Method stability retention time |
Variance components ≤ 5% RSD retention time (buffer strength, additive concentration, column temperature, flow rate, injection volume) |
0.00-2.57%RSD |
Accept |
Post time: Nov-24-2022






